Humatrix, a medical technology company founded in SIP in 2021, announced on June 6 that its LineMatrix?, the first domestically developed bioartificial blood vessel, has gone through the first-in-man (FIM) clinical trial at Sir Run Run Shaw Hospital, Zhejiang University School of Medicine.

The results of the clinical follow-up showed that, after the product was implanted, the primary patency rate in the first three months was 90.9%, with the cumulative patency rate reaching 100%, and that in the first six months was 80.8%, with the cumulative patency rate reaching 100%. The artificial blood vessel did not trigger an immune response in the human body, and no complications such as infection or aneurysm occurred. The performance of this product was significantly better than that of expanded polytetrafluoroethylene (ePTFE) artificial blood vessels, and the clinical effect was excellent.
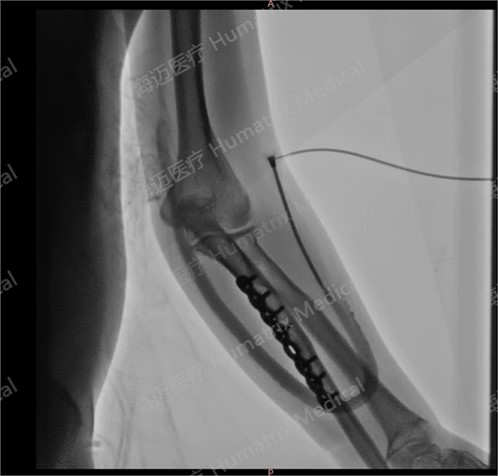
The clinical trial, led by Li Hua, director of Nephrology and Hemodialysis Center at the hospital, involved end-stage renal disease patients undergoing hemodialysis via artificial blood vessels. Since the first implantation on September 24, 2024, all cases had completed follow-up by June 5, 2025, confirming the product’s safety and efficacy in human applications.

June 6, 2025







